Search results
News about COVID-19, LB.1, vaccine
News about SpaceX, Falcon 9, launch
Also in the news
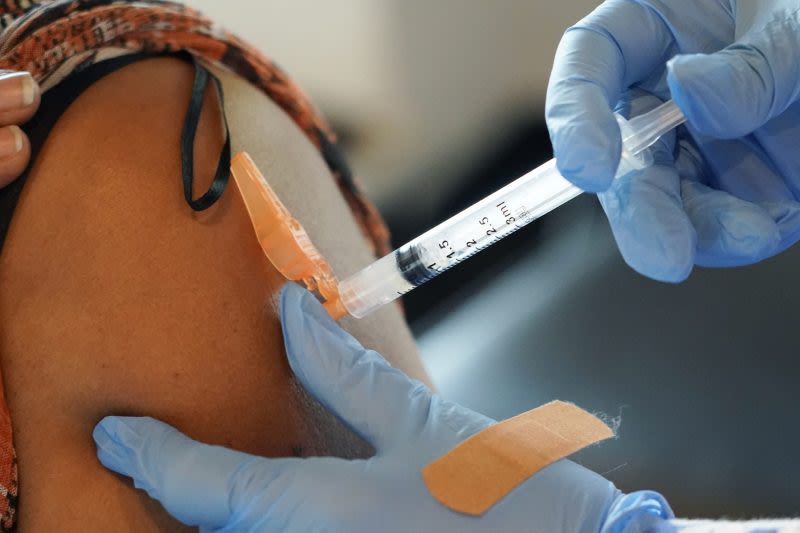
Sep 22, 2021 · Today, the U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered...
- Overview
- How well do these boosters work?
- When should I get the new booster?
- How much will it cost?
- How many boosters will we need?
- What are the side effects?
The Food and Drug Administration earlier this month greenlighted updated Covid boosters from Pfizer and Moderna. The shots, which are formulated to target the XBB.1.5 subvariant, are now available.
Anyone age 5 and older can get an updated booster shot from either Pfizer-BioNTech or Moderna, regardless of whether they were previously vaccinated, the FDA said in a statement on Sept. 11. People who have been vaccinated should wait at least two months after their last Covid shot before getting the new booster.
Novavax, which also makes a Covid vaccine, said on Sept. 11 that its updated booster was still being reviewed by the FDA. Unlike Pfizer and Moderna, Novavax’s shot doesn’t use mRNA technology.
The boosters come as Covid cases are rising in the U.S., driven by a slew of subvariants. Officials hope that the boosters can blunt a possible spike in winter illness.
In June, the FDA asked drugmakers to formulate the fall boosters to target the then-dominant XBB.1.5 subvariant.
While that particular strain is no longer as prevalent, other strains in circulation are still closely related to XBB.1.5.
Two of the predominant strains, EG.5 and FL.1.5.1, are members of the XBB subvariant family. Pfizer, Moderna and Novavax have said that their boosters work against EG.5, and Moderna said its booster also works against FL.1.5.1.
Dr. Eric Topol, executive vice president of Scripps Research in La Jolla, California, said he is “not concerned” about vaccine effectiveness, saying that the vaccines developed so far have consistently shown to provide protection against severe disease, hospitalization and death.
“The new booster will certainly ameliorate protection,” Topol said.
Experts are also keeping a close eye on BA.2.86 — dubbed “Pirola” by some on social media — an omicron subvariant that initially caused alarm because of its high number of mutations. Emerging lab data, however, suggests it may not be immune-evasive or transmissible as initially feared.
For people who haven’t gotten a booster since last fall and haven’t had a recent Covid infection, experts say they should get their booster as soon as possible.
But with rising cases, many people are sick now with Covid or have recently recovered from an infection. According to a CDC official, people with recent infections may wait about 90 days from their illness before getting the booster.
For the first time since the vaccines became available, the federal government will not cover the cost of the shots.
Pfizer and Moderna have said they are pricing each vaccine dose at over $100.
Jennifer Kates, director of the Global Health & HIV Policy Program at the nonprofit KFF, said most people with private and public health insurance should continue to pay nothing out of pocket for the boosters — as long as they stick with an in-network provider.
“If you go out of network, you might have some cost, just like with any other service,” she said.
Most healthy people will likely only need one booster until fall 2024, said Dr. Ofer Levy, the director of the Precision Vaccines Program at Boston Children’s Hospital and a member of the FDA’s advisory committee.
Dr. Paul Offit, a vaccine expert at Children’s Hospital of Philadelphia and another member of the FDA’s advisory committee, said it’s possible people at high risk for severe disease such as older adults or who are immunocompromised may be advised to get an additional dose in a few months.
Levy said the side effects of updated boosters should be the same as the previous iterations of the shots.
Common side effects include headache, chills, fever, nausea and pain or swelling at the injection site, according to the CDC.
Pfizer and Moderna’s vaccines have been associated with a small but increased risk of myocarditis, the inflammation of the heart muscle, mostly in young men. Most people make a full recovery, and early studies suggest incidences of myocarditis are highest following the second dose of a primary series.
Still, Pfizer and Moderna are currently running trials to track health issues — if any — in the years following a diagnosis of vaccine-associated heart problems.
- Berkeley Lovelace Jr.
- 3 min
Sep 13, 2023 · The new boosters are a much closer match to currently circulating variants than prior vaccines, say federal health officials. They're updated versions of the existing Moderna and Pfizer-BioNTech...
May 19, 2022 · Following today’s meeting of the Advisory Committee on Immunization Practices’ (ACIP), CDC is expanding eligibility of COVID-19 vaccine booster doses to everyone 5 years of age and older. CDC now recommends that children ages 5 through 11 years should receive a booster shot 5 months after their initial Pfizer-BioNTech vaccination series.
Sep 1, 2022 · Updated COVID-19 boosters add Omicron BA.4 and BA.5 spike protein components to the current vaccine composition, helping to restore protection that has waned since previous vaccination by targeting variants that are more transmissible and immune-evading.
Sep 28, 2021 · If you’re currently eligible for a booster shot, they are available at 80,000 locations across the nation, and can help you stay healthy and well for the coming holiday season. For others eager to do everything possible to protect themselves, their families, and their communities against this terrible virus—but who are not yet eligible for ...
Jan 4, 2022 · CDC is updating our recommendation for when many people can receive a booster shot, shortening the interval from 6 months to 5 months for people who received the Pfizer-BioNTech COVID-19 Vaccine.