Search results
CVS COVID-19 Vaccine Near Columbus OH
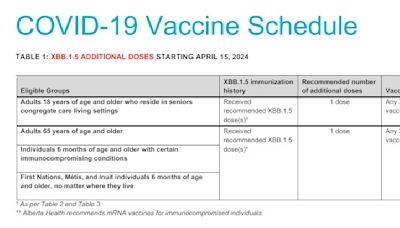
www.cvs.comPharmacy LocationBy appointment only60 North Stygler Road(614) 475-20142680 North High Street(614) 267-56074801 West Broad Street(614) 878-75652532 East Main Street(614) 235-30244280 Morse Road(614) 473-11231400 Parsons Avenue(614) 449-93991717 Olentangy River Rd(614) 298-1078Jul 13, 2023 · COVID-19 Vaccines Are Safe, Effective, and Free. Everyone 6 months and older should get an updated COVID-19 vaccine. People aged 65 years and older who received 1 dose of any updated COVID-19 vaccine (Moderna, Pfizer-BioNTech or Novavax) should receive 1 additional dose of an updated COVID-19 vaccine at least 4 months after the previous updated ...
- Frequently Asked Questions
Yes, COVID-19 vaccination is recommended for people who are...
- Stay Up to Date
Children aged 6 months–4 years need multiple doses of...
- Overview of Covid-19 Vaccines
There are two types of COVID-19 vaccines available in the...
- Your Vaccination
Children aged 6 months – 4 years may need more than 1...
- Myths & Facts
Getting a COVID-19 vaccine is a safer and more dependable...
- Covid-19 Vaccine Effectiveness
Results of vaccine effectiveness studies are critical to the...
- Immunocompromised
COVID-19 Vaccines: 2023-2024 Updated, Bivalent, and Original...
- Frequently Asked Questions
Updated COVID-19 vaccines are now available for children and adults. Availability will continue to increase, so if you don't find vaccines near you, contact your local pharmacy or health care provider, or check back later. Once you find a location that works for you, please confirm vaccine availability through their site.
Nov 3, 2023 · The Novavax COVID-19, adjuvanted vaccine is a protein subunit vaccine. These vaccines include only the parts (proteins) of a virus that best stimulate your immune system. The Novavax COVID-19 vaccine contains harmless S proteins. It also has an ingredient called an adjuvant that helps with your immune system response.
Mar 13, 2024 · January 31, 2022, Spikevax (COVID-19 Vaccine, mRNA), which was previously known as Moderna COVID-19 Vaccine; the approved vaccine will be marketed as Spikevax for the prevention of COVID-19 in individuals 18 years of age and older. December. December 10, 2021 HHS Secretary issued a to receive a booster dose of the Pfizer COVID-19 vaccine.
Novavax COVID-19 Vaccine, Adjuvanted: On July 13, 2022, the FDA reauthorized emergency use of this two-dose vaccine series for people age 12 and older. This is the first protein-based COVID-19 vaccine to receive FDA authorization and is similar in design to vaccines against the flu and shingles.